7) STATISTICAL PROBLEMS II
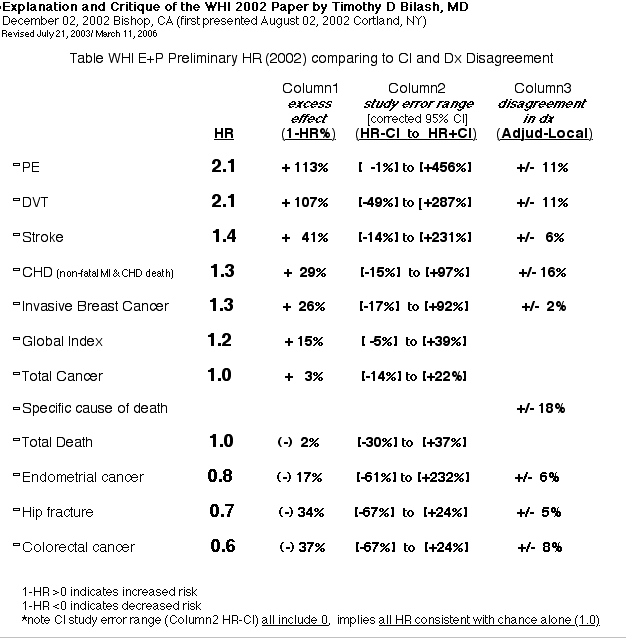
- dont know what percentage of adverse events (ie., PE/DVT) were confirmed by imaging/ lab studies as opposed to clinical diagnoses
- "Overall CHD rates were low" in the study compared to the general population.
- most excess was in nonfatal MI
- this supports a diagnosis bias or a population bias (affects CHD conclusions)
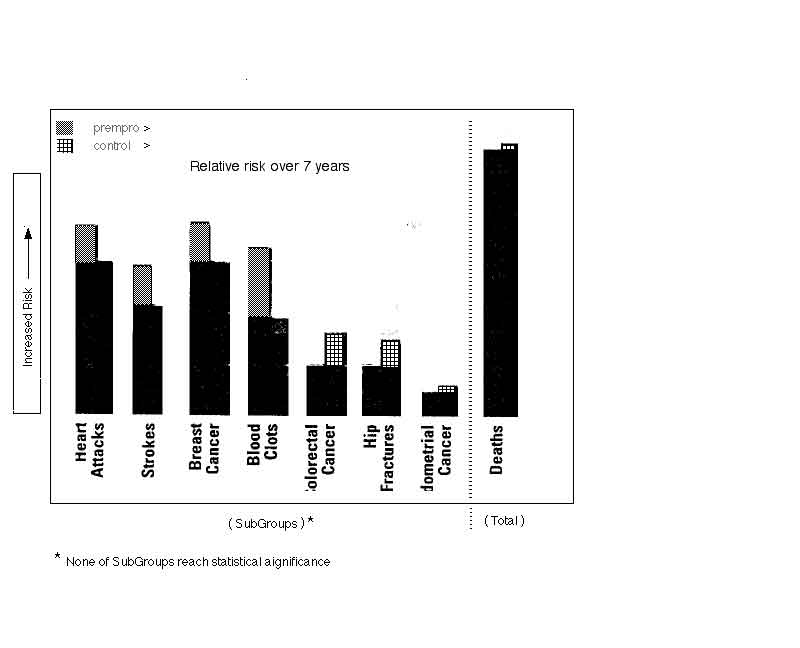
- No trends were seen
- there are no statistical trends over the seven years for incidence of disease:
- there was no difference in mortality or cause of death between the groups
- [see Table 3]
- however, there is a trend after 5 years showing decreased death rate, just as meaningful as the incidence data
- [see figure 4]
8) Grouping and Outcome Inaccuracies
Summary of inaccuracies inherent to the study:
- 04% of prempro arm had received estrogen only in the beginning of the study
- 01% switched groups post hysterectomy
- 42% discontinued active treatment
- 35% additional patients were unblinded, additional 11% crossover to active treatment
- 05-16% discrepancy in diagnoses depending on diagnosis
- 03% died
- 03% lost to followup
- Only 8% of enrolled patients were left in the study after 7 years (1,300 of 16,000)a*
9) Life Table Analysis Limitations
"Enron" Accounting ???
- Experimental Results (compares numbers)
- Life Table Analysis (compares rates)
- Signal vs Noise
- This publication was published in JAMA with no author, prior to expected publication
10) Hormones and Menopause
- Hormones
- FSH
- LH
- Estrogen (E2)
- Estrone (E1)
- Progestins
- Progesterone
- Provera (medroxyprogesterone acetate)
- Norethindrone
- Newer ones
- Steroid interconversion diagram
- Free hormone is the active form, not total hormone
- Hormone changes in menopause
- run out of eggs in ovary, so make less estrogen (E2)
- E2 starts decreasing at 38 years
- estrogen (E2) source shifts to estrone (E1)
- converted from adrenal androgens (primarily androstenedione) in adipose
- not from ovary
- gradual rise in FSH in response to low estrogen and low inhibin
- lower sex hormone-binding globulin (causes more free estrogen and free testosterone)
- HRT Side Effects
- distinguish Nuisance vs Harm
11) Breast Cancer Biology
- Does it make sense?
- medroxyprogesterone acetate slows breast cancer lines positive for receptors in humans
- progesterone reduces cell divisions in breast
- progesterone decreases estrogen (progesterone) receptors
- progesterone stimulates 17beta-dehydrogenase (converts to estrone)
- estrogen induces progsterone receptors
- control of cellular response of breast epithelium appears unrelated to direct effects of estrogen and progesterone
- progesterone causes down-regulation of estrogen receptors in luteal phase (2nd E2 receptor pool shows no effect)
- in vivo and in vitro studies conflic
- there are two kinds of progesterone receptors giving varied responses depending on the tissue
- model: progesterone promotes start gene or stop gene depending on target tissue and timing of presence
12) Other Studies/ Epidemiology/ Animal Studies
- EPIDEMIOLOGY
- no increased risk with increased progesterone dose
- increased incidence confined to in-situ tumors (reverse of WHI)
- breast feeding protection may be due to bias- needs further study
- increased screening, increased diagnosis, increased survival (?)
- Iceland- incidence has increased, mortality rates stable
- Norway- increase in incidence has slowed now
- Sweden- cohorts by age showed no increase risk from OC's
- ANIMAL STUDIES
- in rats
- estrogen promotes tumors, progesterone inhibits tumors
- pretreatment with progesterone prevents or decreases growth promotor effect of estradiol
- OTHER (OBSERVATIONAL) STUDIES
- results are mixed, three studies indicate increased risk
- increased breast cancer risk from ERT runs 1.3 - 2 fold
- primarily in subgroup with alcohol consumption
- late natural menopause increases risk
- see Table C
- increased risk of breast cancer diagnosis, not risk of breast cancer mortality
- London Nurses Health Study (increased risk current users, but not with duration of use)
- Risk-benefit of HRT (model calculations, England)
- Greatest impact found on ischemic heart disease
- prevention of death (see figure D), gain in life span
- assumes breast cancer RR=1.3
- gains wiped out if RR=2 (but not worse)
- increased hospitalization?
- CASH data shows increased risk < 35 yrs, lower risk 45-54 yrs, but lower mortality
- HERS (Heart and Estrogen/Progestin Replacement Study)
- 2763 all had CHD
- early increased risk with no overall increase after 5 years
a*(Added 02.08.2005) The 2002 paper for Kaplan Meier Analysis indicates approximately 1300 patients remaining after 7 Outcome years [~800 Estrogen-Progestin/~500 Placebo depending on Outcome, Fig3&4 p328&329]. Censoring criteria are not the same for Cox Proportional Hazard Analysis, which indicates 9000 patients left [5129 Estrogen-Progestin/4243 Placebo, Table 4 p329]. The 2002 paper (as well as subsequent reports) have made no effort to clarify such issues, often placing critical information only in the small print subtext of figures, or not including any comment at all.
back
DrTim homesite
 page views since Sept2007
page views since Sept2007